Photo Credit: by @CANPharmacyWorld
What’s in a name? If you’ve never wondered about how pharmaceutical drugs get their names, well…you’re not alone. It’s not our daydream of choice either.
But if you’ve never felt absolutely astounded by the sheer cost of naming a prescription drug, then you simply don’t know enough about the industry. The pharmaceutical industry is one of the largest worldwide, and various elements of the industry -- drug development included -- involve astronomical costs that would shock even the most business savvy. How much money do you think it costs to bring a drug to market? What about how much it costs simply to name the drug? It’s no small change, that’s for sure: we’re talking seven figures here.
There’s an entire science behind naming prescription drugs. Companies have to consider the creation and development process of the drug, figure out their costs and profit margins, and of course, successfully brand and market the drug so that it enjoys a long and profitable life span on the market. Every step of the way further complicates the process -- and makes it all the more expensive. Factoring all of that may sound like a lot, and it is. That’s likely why such high costs are involved. This industry certainly is not for sissies!
For an inside look at how the pharmaceutical industry approaches and releases new prescription drugs on the market, read on. We’ll look into the fascinating name creation process and take a look at considering the costs of this part of the business.
It costs HOW much to name a prescription drug?!
According to StickyBranding, in an article aptly titled “The cost of naming prescription drugs is insane,” we learn that “Pharmaceutical companies are spending big bucks to name their drugs. An average naming project costs $250,000, and that doesn’t include the costs of registering, trademarking, and getting the name approved in the various regulatory bodies. At the end of the project, pharma companies can spend up to $2.5 million on a brand name. Those costs might seem outrageous, but they are a symptom of a high stakes game and a diminishing resource.”
How does this make sense? We’ll explore more below.
Let’s start at the beginning. In short: yes, a lot of money is invested into the mere naming of a prescription drug. But the cost of that pales in comparison to how much money is invested into the development of a drug in total, from start to finish. According to Scientific American, it costs over $2.5 billion to bring a drug to market (and that price has doubled in the past ten years alone). So when you realize how much money is invested into one drug that actually meets market approval and is available for sale, you realize that the cost of naming a drug is simply proportionate to the cost overall of the new drug’s entrance into the market.
Of course, most of those multiple billions of dollars go into the testing and development phase of the drug. Most new medications and drugs being tested never even make it to market, for one reason or another, usually because they are not safe or effective enough to meet consumer need. Still, the cost of testing and developing those products adds up. For the small percentage of drugs that do make it to the market, researchers and pharmaceutical companies then need to make up for all the costs they incurred on the failed drugs.
The article continues, “The amount spent to develop any individual drug depends mostly on what it costs to conduct studies to prove it is safe and effective and secure regulatory approval [more on that below]. That can range from $10 million to $2 billion, depending on what the drug is for. But what really drives up costs is the fact that 90% of medicines that start being tested in people don’t reach the market because they are unsafe or ineffective. The $2.7 billion figure includes the cost not only of these failures, but also of not putting the money spent on them into something that would give a more reliable return.”
This is part of the reason that pharmaceuticals deal in such high numbers; the drugs that make it to market have to pay off enough to make up for all the time and cost invested in the many drugs that did not make it to market. Thus, investing just a small amount of the overall cost into getting the name right doesn’t seem so crazy or outrageous anymore.
The pharmaceutical drug naming process
In addition to the costs and factors above, the naming process isn’t so easy. The majority of proposed drug names are rejected. This is mostly due to the potential for confusion with other drugs on the market. With a flooded market of thousands of pharmaceutical drugs, it can be hard to come up with something original! It’s important to get the name right and ensure that it is unique and could not be mixed up with another drug. That’s why pharmaceutical companies are willing to invest so much into getting the name of the drug right.
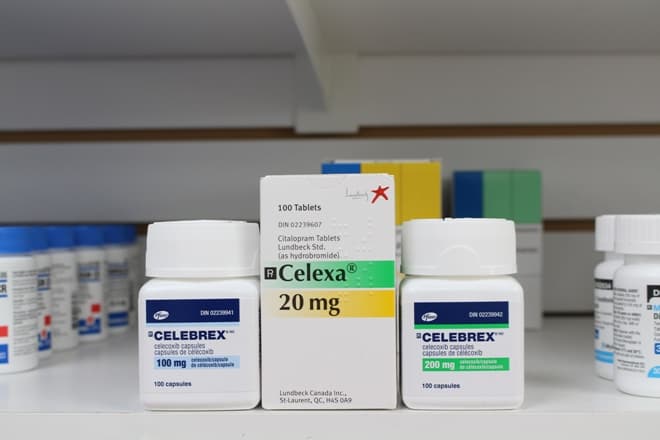
For example, what if you mixed up Zantac (a heartburn drug) with Xanax, a medication used to treat anxiety? Or how about Celebrex (a drug for pain and inflammation) and Celexa (an antidepressant), Lamisil (an antifungal cream) and Lamictal (a drug for epilepsy and bipolar disorder)? These could have serious, even life threatening, implications if they were mixed up or prescribed improperly due to simple human error. Drugs that are spelled similarly or sound the same can lead to serious problems for patients -- and serious, costly lawsuits for drug makers. It’s best to avoid these risks altogether by spending that money upfront to invest in the right name that will not cause any trouble down the road.
And, of course, get the name right and you’ve got a product that’s positioned to become a smash success. Take Advil, for example. How much more are you willing to pay for a branded bottle of Advil than a generic bottle of ibuprofen? If you’re anything like the average consumer, the answer is quite a lot more. In fact, Advil is typically double the cost of a generic version of the same drug.
Creating an entire brand around the name of a drug can help people remember it, ask for it from their doctors, buy it, and use it. At the end of the day, that’s what pharmaceutical companies want. Become a household name or the trusted brand that people turn to for whatever prescription they need, and you’ve got yourself a successful, profitable drug that will continue to provide returns. It helps you stand out and weaves a story around your brand, positioning you as the trusted source of the drug.
For all of these reasons and more, naming a drug -- and doing it right -- is essential to the success and long term profit margins of a drug. Investing a few hundred thousand dollars into something that will have a multi-million dollar life span, something that will endure in perpetuity, no longer seems like such a sacrifice or a major cost in the grand scheme of things. It all comes down to the numbers. What provides a good return on investment is what will be invested into, and that’s just good business sense.
Why is it getting more expensive to create prescription drugs?
One thing we mentioned above may have caught your eye. The cost of getting a new drug to market is high, yes -- but what’s even more surprising than the high cost is that the cost has doubled, and has done so in the past ten years alone. What gives? Why is it becoming so much more expensive to come out with new prescription drugs?
Scientific American says that “The steep rise in costs comes despite an intense effort in recent years to bring efficiency to pharmaceutical R&D [research and development]. Offsetting any such savings … are higher costs due to the increased complexity of clinical trials, a greater focus on chronic and degenerative diseases, and tests for insurers seeking comparative drug effectiveness data. Noting the high cost of failure in drug development … the cost of unsuccessful projects [must be] figured into [the] group’s analysis.”
Some believe that drug pricing should be based not on how much the drug cost the company during the research and development phase, but on how much value a certain drug delivers to those who take it or rely on it. This makes sense, but maybe not for the pharmaceutical companies. After all, they have to look after their bottom line. It’s not the consumer’s job to make sure that the researchers have the money they shelled out to develop a new drug returned to their pockets at the market’s earliest convenience. That’s the company’s problem, and that’s what they’re in business to do.
It’s controversial, but big pharmaceutical companies know that people will pay a high price -- may even any amount -- for a drug that will save their lives, save the lives of their family members, or simply make their life all the more pleasant and convenient. Epi pens have hit headlines in recent years for their soaring costs. But what highly allergic person won’t shell out their hard earned money to ensure they’ll live to see another day? What cancer patients won’t pay whatever it takes to be cured of their disease?
At the end of the day, pharmaceutical companies are well aware that the general public needs them. They’re well aware that many people simply wouldn’t survive without them. Thus, like it or not, they’re able to charge whatever they feel is appropriate and financially rewarding. They can exploit the nature of their line of work or they can choose to set a fair price. Fortunately the government intervenes in this area and places some degree of regulations, but ultimately, people will likely always face a high price tag for the trustworthy, effective, recognizably branded drugs and medications they need.
How do pharmaceutical industries name new drugs? What happens when a new drug has been named?
Wait a minute, so then where does the generic name of a drug come from? We’re glad you asked!
No, these drug substance combinations do not simply exist out there waiting to be discovered, like math. They don’t come with a pre existing name or label. Instead, companies must come up with a name for them. Once all of the initial process is over with and the new drug is ready for release and the name has been decided and all systems are go… well, now what? With all of this talk about new drugs, what about the less expensive generic drugs -- how do those work? We’ll fill you in.
According to Merck Manuals, “Drugs often have several names. When a drug is first discovered, it is given a chemical name, which describes the atomic or molecular structure of the drug. The chemical name is thus usually too complex and cumbersome for general use. Next, a shorthand version of the chemical name or a code name (such as RU 486) is developed for easy reference among researchers.
When a drug is approved by the Food and Drug Administration (FDA—the U.S. government agency responsible for ensuring that drugs marketed in the United States are safe and effective), it is given a:
-- Generic (official) name
-- Brand (proprietary or trademark or trade) name
… The generic name is assigned, in the United States, by an official body—the United States Adopted Names (USAN) Council. The brand name is developed by the company requesting approval for the drug and identifies it as the exclusive property of that company. When a drug is under patent protection, the company markets it under its brand name. When the drug is off-patent (no longer protected by patent), the company may market its product under either the generic name or brand name.”
Even then, the drug companies must make sure the brand name and generic name of the drug are not too similar so that the pharmacy workers do not accidentally get the prescription drugs messed up.
Looking for brand name or generic prescription drugs? We’ve got both. We can help you out! We offer both kinds of drugs at great prices. Check out our full product lineup and see if your prescription is available to order today.
###
Skye Sherman is a professional writer who has been published in numerous local and international outlets. She has also worked for a wellness company and is very familiar with the healthcare industry. She holds a degree from a Florida university.
Your email address will not be published. Required fields are marked with *.